Explain Why Different Elements Each Produce a Unique Line Spectrum
Atomic Spectra We know that when elements or their compounds are heated they release energy in the form of light which gives rise to a line spectrum. The spectra for each element are unique because each element contains differing numbers of electrons and thus different energy levels.
Spectroscopy In Astronomy Astronomy
Each element has a different number of electrons and this different number leads to an unique ground state for these electrons.
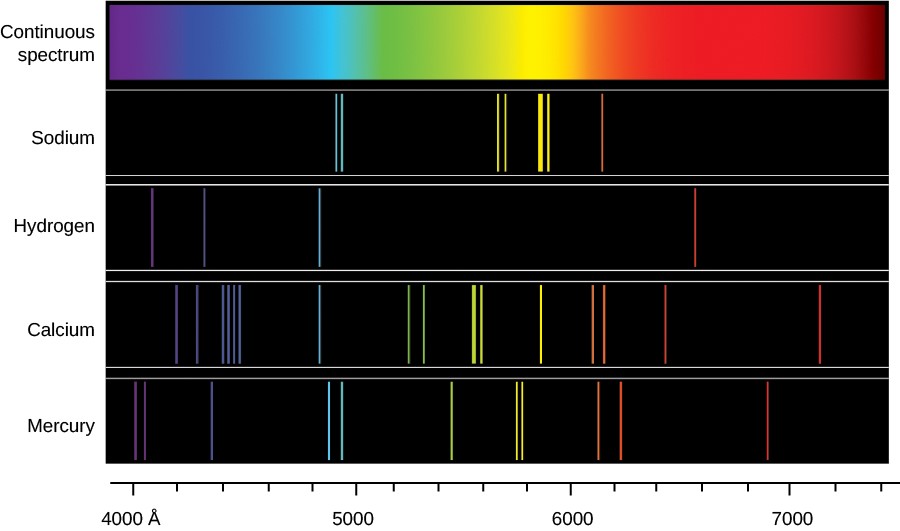
. Each element has a different set of emission colors because they have different energy level spacings. However sunlight has a combination of other elements like helium and hydrogen as evident in the absorption gaps seen between the rainbow colors. Each element has its own unique atomic emission spectrum.
This means that line spectra can be used to identify elements. Subsequently question is what does each line in the. When a gas is cool it absorbs the same wavelengths of light as it would emit when it is hot.
The suns light is also believed to produce continuous spectrum since we can see the rainbow. The lights travel on different line of spectrum that produce different color. Atoms of individual elements emit light at only specific wavelengths producing a line spectrum rather than the continuous spectrum of all wavelengths produced by a hot object.
The lines in the sodium lamp are broadened by collisions. The emission lines correspond to the differences between various pairs of the many energy levels. There are many possible electron transitions for each atom.
Thus we can make different colors from elements that produce various colors. Due to the availability of multiple states of energy an electron can undergo numerous transitions each giving rise to a unique wavelength that comprises the emission spectrum. Each element has its own unique line spectrum and is thus referred to as the fingerprint for a particular element.
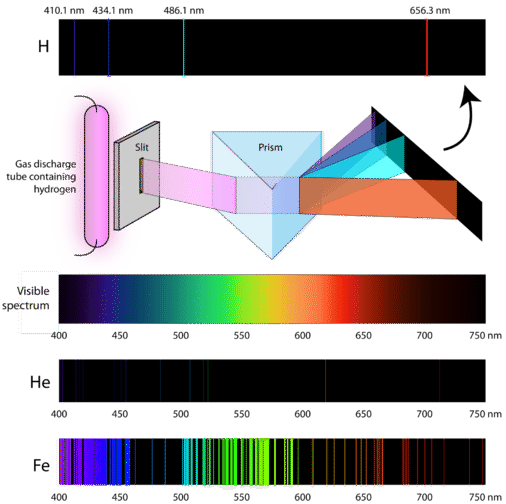
You can look at the spectra and identify which elements are present. The lines photons are emitted as electrons fall from higher energy orbitals to lower energies. Also why do different elements produce different spectra.
The emission lines correspond to the differences between various pairs of the many energy levels. In other words an atomic spectrum can be used as a fingerprint for an element because it is unique for each element and reflects the energy levels occupied by the. Different elements emit different emission spectra when they are excited because each type of element has a unique energy shell or energy level system.
Different elements have different flame colours because their electrons have different allowed energy levels. When you heat an atom some of its electrons are excited to higher energy levels. The emission lines correspond to the differences between various pairs of the many energy levels.
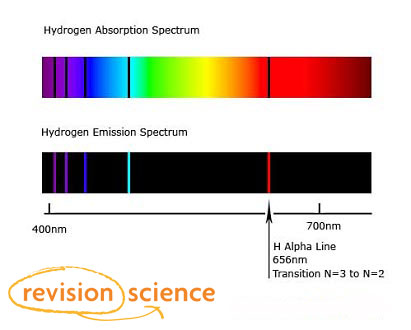
Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed. The lines photons are emitted as electrons fall from higher energy orbitals to lower energies. The Bohr model says that electrons exist only at certain allowed energy levels.
Each element has its own unique line spectrum and is thus referred to as the fingerprint for a particular element. Due to the very different emission spectra of these elements they emit light of different colors. As the number of electrons in an atom increases it will have a large number of emission and hence the element with maximum no.
Answer 1 of 4. As a result each produces photons with different energy and so the line spectra for different elements will be different. Each elements emission spectrum is distinct because each element has a different set of electron energy levels.
The lines photons are emitted as electrons fall from higher energy orbitals to lower energies. Each jump corresponds to a particular wavelength of light. Then they jump back down again.
Each elements emission spectrum is distinct because each element has a different set of electron energy levels. The emission lines correspond to the differences between various pairs of. A gas cloud on its own without a light source behind it produces a line emission spectrum.
What the spectral lines show is the transition from an excited state of theses electrons to a return to the ground state. Of an electron or the last element of the periodic table will produce lar the est number of spectral lines. Different elements have different spectra because they have different numbers of protons and different numbers and arrangements of electrons.
Elements produce different line spectras because they are exposed to heat and they give off a colour or line spectra. When an atom absorbs energy its electrons jump to higher energy levels. The dark line in the center of the high pressure sodium lamp where the low pressure lamp is strongest is cause by absorption of light in the cooler outer part of the lamp.
Because different elements react differently to heat. Since each element has its own unique electron arrangement the light that is emitted by the atoms produces an emission spectrum that can be used to identify the element. Each elements emission spectrum is distinct because each element has a different set of electron energy levels.
That is why that when the energy level rises from the excitement to heat the element begins to produce the light according to its reaction to the energy level and line of spectrum. The mercury in a florescent lamp emits a. The differences in spectra reflect the differences in the amount of energy that the atoms absorb or give off when their electrons move between energy levels.
The spectra for each element are unique because each element contains differing numbers of electrons and thus different energy levels. When any element is excited to the point where it emits visible light it emits a unique spectrum. Truly white light like that emitted by some stars and moons is a perfect example of continuous spectrum.
Each elements emission spectrum is distinct because each element has a different set of electron energy levels.

Formation Of Spectral Lines Astronomy
Comments
Post a Comment